ARDS를 동반한 코로나-19 환자에 대한 혈장치료 국내 성공사례(연세의대, J Korean Med Sci 발표)
첨부파일
-
- 첨부파일: plasma.jpg (262.7K)532
짧은주소
본문
ARDS를 동반한 코로나-19 환자에 대한 혈장치료 국내 성공사례(연세의대, J Korean Med Sci 발표)
Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea
J Korean Med Sci. 2020 Apr 13;35(14):e149. English.
Published online Apr 06, 2020.
Jin Young Ahn,1,* Yujin Sohn,1,* Su Hwan Lee,1 Yunsuk Cho,1 Jong Hoon Hyun,1 Yae Jee Baek,1 Su Jin Jeong,1 Jung Ho Kim,1 Nam Su Ku,1 Joon-Sup Yeom,1 Juhye Roh,2 Mi Young Ahn,3 Bum Sik Chin,4 Young Sam Kim,1 Hyukmin Lee,2 Dongeun Yong,2 Hyun Ok Kim,2 Sinyoung Kim,2 and Jun Yong Choi1
1Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
2Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
3Department of Internal Medicine, Seoul Medical Center, Seoul, Korea.
4Department of Internal Medicine, National Medical Center, Seoul, Korea.
▷Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 not yet has established its treatment, but convalescent plasma has been expected to increase survival rates as in the case with other emerging viral infections. We describe two cases of COVID-19 treated with convalescent plasma infusion. Both patients presented severe pneumonia with acute respiratory distress syndrome and showed a favorable outcome after the use of convalescent plasma in addition to systemic corticosteroid. To our knowledge, this is the first report of the use of convalescent plasma therapy for COVID-19 in Korea.
▷INTRODUCTION
An outbreak of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, which began in Wuhan, China, has emerged as a primary concern all over the world. The World Health Organization announced the risk assessment of coronavirus disease 2019 (COVID-19) as very high at the global level, and fatal cases are rapidly increasing. By March 24, 2020, more than 9,000 people were infected, and 126 people died, in South Korea. Without specific treatments for the virus, treatment options are being studied in addition to supportive care. Evidence shows that the use of convalescent plasma to treat emerging viral infections, including SARS, Middle East respiratory syndrome (MERS), Ebola virus disease or avian flu, can improve survival rates in patients whose condition worsens even with conventional treatment.1, 2 However, the safety and efficacy of convalescent plasma treatment in COVID-19 have not been known. Here, we report two cases of severe COVID-19 patients presenting acute respiratory distress syndrome (ARDS), who showed a favorable clinical course after the convalescent plasma infusion. This is the first report of the use of convalescent plasma to treat cases of SARS-CoV-2 infection in Korea
▷CASE DESCRIPTION
Case 1
A previously healthy 71-year-old man visited the Community Health Center on February 22, presenting 12 days of fever and cough. He underwent an examination of SARS-CoV-2 via real-time reverse transcription polymerase chain reaction (rRT-PCR) and diagnosed as COVID-19. He admitted to the local public medical center and 400 mg of hydroxychloroquine once daily was started. A chest radiograph obtained on day 2 showed mild opacities in the right lower lung, lopinavir/ritonavir 400 mg/100 mg twice daily was added. However, on day 3, oxygen demand increased, so he transferred to the tertiary-care hospital.
At the time of arrival, the patient had no subjective dyspnea under 4 L/min oxygen flow via nasal cannula, but the respiratory rate was over 30 times per minute. Chest radiographs demonstrated rapidly aggravated bilateral infiltration. Routine blood tests found white blood cell (WBC) count at 3.53 × 103/µL, with lymphopenia of 0.4 × 103/µL. C-reactive protein (CRP) and lactic dehydrogenase (LDH) elevated up to 59.7 mg/L and 814 IU/L. Routine chemistry, electrolyte, and blood coagulation tests revealed no abnormalities except mildly elevated aspartate transaminase. The level of interleukin 6 (IL-6) was increased as 101.3 pg/mL. Serial bacterial culture and polymerase chain reaction (PCR) for other respiratory viruses were all negative.
Intubation and mechanical ventilator care were started according to the management of ARDS. Despite the continuous use of lopinavir/ritonavir, hydroxychloroquine and empirical antibiotics, he remained febrile with aggravated oxygenation profiles and chest images. Laboratory test showed further elevation of CRP (172.6 mg/L), IL-6 (208.2 pg/mL).
On day 9, the arterial blood gas analysis showed PaO2/FiO2 of 86, consistent with severe ARDS. Intravenous methylprednisolone (1 mg/kg/day daily) was started. On day 10, convalescent plasma was obtained from a male donor in his 20s who had recovered from COVID-19 for 21 days. He was diagnosed as COVID-19 presenting fever, cough and pneumonia, however, showed complete recovery and didn’t have any symptom at the time of plasma donation. He has met the blood donor eligibility criteria for plasma donation, including age, weight, reasonable-sized antecubital veins. Also, allogeneic donor screening tests, defined by enforcement rules of the Blood Management Act in Korea, were acceptable for transfusion. Donor apheresis was performed with Spectra Optia apheresis system (CMNC software; Spectra Optia IDL Tubing set; Terumo BCT, Lakewood, CO, USA), 500 mL of convalescent plasma was collected. Anti-SARS-CoV-2 IgG antibody in plasma was measured by enzyme-linked immunosorbent assay (ELISA) (Novel Coronavirus COVID-19 IgG ELISA kit; Epitope Diagnostics, San Diego, CA, USA) and optical density (OD) ratio for IgG was 0.586 (cut-off value 0.22). The plasma was divided into two doses and administered to the patient at 12 hours interval. Each dose was given over for 1 hour. No adverse reaction occurred after the administration of convalescent plasma.
The fever subsided, and oxygen demand decreased since day 11. The patient's condition much improved with decreased CRP and IL-6 to normal range (5.7 mg/L and < 1.5 pg/mL, respectively), and on day 18, PaO2/FiO2 increased up to 300 (Fig. 1). A chest X-ray revealed further resolution of both lung infiltrates (Fig. 2). SARS-CoV-2 was quantified by detection of the RNA-dependent RNA polymerase region of the ORF1b gene on rRT-PCR, the value of cycle threshold (Ct) changed from 24.98 on day 10 to 33.96 on day 20 after plasma infusion (Fig. 1). SARS-CoV-2 was negative after day 26. The patient underwent a tracheostomy and currently, is successfully weaned from the mechanical ventilator.
Case 2
A 67-year-old woman with a medical history of hypertension developed fever and myalgia and diagnosed as COVID-19 via SARS-CoV-2 rRT-PCR on March 6. The next day, she was admitted to a local public medical center and received hydroxychloroquine 400 mg once daily and lopinavir/ritonavir 400 mg/100 mg twice daily with empirical antibiotics. However, on day 3, she was transferred to the tertiary-care hospital due to increased oxygen demand and worsening infiltrative shadows in the left lower lung. At that time, her oxygen saturation checked 93% on 4 L/min oxygen flow via nasal cannula with a respiratory rate of 24 times per minute. Routine blood tests showed mild leukocytosis (12.67 × 103/µL) with lymphopenia (0.7 × 103/µL), elevated CRP, IL-6 and LDH. (131.1 mg/L, 474.7 pg/mL, 344 IU/L, respectively) Routine chemistry, electrolyte, and blood coagulation tests showed no abnormalities. Bacterial cultures and the PCR for other respiratory viruses were all negative.
She received high flow oxygen therapy but bilateral infiltration and oxygenation were deteriorated, so intubation and mechanical ventilator care started on day 4. Intravenous methylprednisolone (0.5 mg/kg/day daily) was also added. She had sustained high fever with rapidly increasing CRP (314 mg/L), WBC (21.79 × 103/µL), and persistent lymphopenia (0.5 × 103/µL). PaO2/FiO2 fell to 76, consistent with severe ARDS. After applying for the prone position according to the management of ARDS with the use of steroids, chest images and the oxygen demand began to be improved.
On day 6, convalescent plasma was obtained from a male donor in his 20s who had recovered from COVID-19 for 18 days. He was diagnosed as COVID-19 presenting fever, cough and pneumonia however, showed complete recovery and serial PCRs for SARS-CoV-2 were all negative after hospital discharge. Donor screening and plasma collection were performed as mentioned above in the Case 1. OD ratio for IgG was 0.532 and the plasma was administered to the patient in the same way as Case 1. There was no adverse reaction during the plasma transfusion. Leukocytosis and lymphopenia were immediately recovered after convalescent plasma infusion. On day 9, the density of bilateral infiltration on chest X-ray much improved with increased PaO2/FiO2 to 230. The level of CRP and IL-6 also recovered to the normal range (Figs. 3 and 4). SARS-CoV-2 was quantified by rRT-PCR; the value of Ct changed from 20.51 on day 5 to 36.33 on day 9 after plasma infusion (Fig. 3). The patient is successfully extubated and discharged from the hospital on day 24. SARS-CoV-2 was negative after day 20.
▷DISCUSSION
The study of the therapeutic benefits of plasma transfusion of a cured person from infectious diseases began in the 20th century.2 As new antibiotics, antiviral agents, and vaccines are developed, administration of convalescent plasma is not a common treatment, but it can be still an important treatment in the absence of specific treatment of new infectious diseases.3 Over the decades, convalescent plasma has proved its effectiveness as a potential treatment in patients with MERS-CoV,4 H1N15 and H5N1 avian flu,5 and SARS-CoV.6 A systemic review and meta-analysis to evaluate the clinical effects of convalescent plasma shows a statistically significant reduction of mortality.1 In this context, convalescent plasma can be a promising treatment option for severe COVID-19 patients.
As can be seen in the two cases, both received lopinavir/ritonavir and hydroxychloroquine but showed persistent fever, rapidly aggravated hypoxemia and progressive bilateral infiltrations in accordance with the criteria of severe ARDS. After convalescent plasma infusion, the patients showed improved oxygenation and chest X-rays with decreased inflammatory markers and viral loads.
Intravenous methylprednisolone was started just before the convalescent plasma infusion in both cases. We did not use corticosteroids from the beginning as a routine treatment. Current guidelines recommend that systemic corticosteroids should not be given routinely for the treatment of COVID-19 due to the lack of evidence of its clinical efficacy on mortality reduction.7, 8 However, we decided to start corticosteroids when the patients' condition rapidly deteriorated to ARDS. Methylprednisolone was administered one day and two days before the plasma infusion in case 1 and case 2, respectively. Serial laboratory and oxygenation parameters showed rapid improvement right after the corticosteroid administration even before the convalescent plasma infusion.
ARDS is partly caused by cytokine storm and host immune responses.7 Autopsy of patients dying from COVID-19 shows diffuse alveolar damage with exudate and inflammation very similar to those seen in SARS and MERS-CoV infections.9 Theoretically, systemic corticosteroids may have a role to dampen excessive lung damage due to inflammatory responses.10 The recent article about risk factors associated with ARDS and death among COVID-19 patients showed that treatment with methylprednisolone might be beneficial to reduce the risk of death for patients developing ARDS.11 However, corticosteroids are also thought to inhibit proper immune responses and viral clearance and delay antibody production.12, 13
Convalescent plasma infusion might play a role in the coexistence of benefits and concerns of corticosteroid use. Antibodies contained in the convalescent plasma will suppress viruses.14 In an animal study, passively transferred antibodies can provide total protection as well as the maintenance of high levels of antibody titer until the host's immune responses could be increased to clear the viral infection. Besides, in vivo studies showed that the effects of neutralizing antibodies were not only limited to viral clearance, but also included acceleration of infected cell clearance.15
In our cases, the viral load estimated by Ct values showed an increasing trend just before plasma infusion but began to decrease right after the use of convalescent plasma. Although improvement of inflammatory marker and oxygenation could be contributed to the combined use of corticosteroid, decreased viral load of SARS-CoV-2 might mean the effectiveness of convalescent plasma in the treatment of COVID-2.
Convalescent plasma was administered after 22 days from the onset of symptoms in Case 1, and 7 days in Case 2, respectively. Because these are not in the early phase of the disease, it is difficult to determine clearly that the decrease in the viral load shown in both cases is due to convalescent plasma or natural pathology of COVID-19. Other studies about viral kinetics of COVID-19 show naturally reducing viral titers after 7–10 days from onset in most patients.16, 17 However, Liu and colleagues reported that severe patients requiring intensive care unit admission due to COVID-19 had high viral load for a longer period than in mild patients.16 Both our cases presented severe ARDS and the viral loads were in increasing trend at the time of plasma infusion regardless of the date of onset.
In Case 2, the patient showed lymphopenia from day 1, and it persisted even after clinical improvement with corticosteroid use. When the convalescent plasma was administered on day 6, lymphocyte count immediately rose to normal level (from 0.52 × 103/µL to 1.21 × 103/µL) and then remained in the normal range. Patients with severe COVID-19 pneumonia and ARDS also presented with lymphopenia in other studies.11 Some authors hypothesized that continuous and gradual increases in lymphocyte count might be required for immunity against SARS-CoV-2 infection.11 In SARS patients, lymphopenia existed at the onset of illness and persisted until the recovery period.18, 19 These findings were consistent with the recovery of lymphocyte count with clinical improvement of Case 2 after the use of convalescent plasma.
We could not assess neutralizing antibody titers from the convalescent plasma. Plasma with high neutralizing antibody titers is likely to be available from the patients in the convalescent phase recovered from severe infection.20 To use plasma for treatment, a neutralization test is suggested as the optimal assay for assessing proper donor or plasma. However, some studies showed that ELISA IgG correlates well with neutralization titers in MERS cases so that it might be a suitable screening test for plasma donation.20, 21 In our cases, donors presented bilateral pneumonia in the course of COVID-19 and both showed positive results in the ELISA IgG test for SARS-CoV-2.
There are still limitations for the use of convalescent plasma. Scientific evidence is insufficient due to the lack of large-scale clinical trials that may be representative of the target populations. Second, the number of antibodies administered to each patient was not standardized. Finally, convalescent plasma usually proceeds with other treatments, such as antiviral agents and steroids, which can affect the relationship between convalescent plasma and antibody, confounding the results.
Despite the limitations, our cases suggest that convalescent plasma from patients who have recovered from COVID-19 infection might be an additional option to treat patients without causing any severe adverse effects. Also, when used with systemic corticosteroids, we might expect the possibility of reducing excessive inflammatory response by corticosteroids as well as promoting the reduction of viral loads by convalescent plasma simultaneously. Further well-designed studies are needed to demonstrate the efficacy and safety of convalescent plasma transfusion in COVID-19 patients.

























댓글목록
대피연님의 댓글
美 FDA, 코로나19 환자 '혈장치료' 지침 공개
http://m.biospectator.com/view/news_view.php?varAtcId=10049
대피연님의 댓글
[중대본]코로나19, 회복기 혈장 채혈지침 마련, "환자 치료는 논의"
방역당국이 코로나19 치료를 위한 회복기 혈장 채혈지침을 마련했다.
단, 혈장치료를 임상 현장에서 어떻게 활용할지에 대한 논의는 진행 중이다.
정은경 중앙방역대책본부장은 13일 충북 오송 질병관리본부에서 열린 정례브리핑에서 "회복기 혈장 채혈지침을 완성했으며, 의료기관 혈액원으로 이미 그 내용을 공유했다"고 말했다.
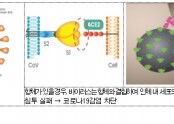
혈장 치료는 바이러스 등에 감염됐다가 완치한 사람의 혈액에 항체가 생긴다는 점을 이용한 치료법이다. 완치자 혈액에서 바이러스를 무력화하는 '중화항체'가 담긴 혈장을 분리해 환자에게 수혈하듯이 제공하는 것이다.
국내에서는 2015년 메르스(MERS·중동호흡기증후군) 환자 치료를 위해 회복기 혈장을 사용한 적이 있다. 최근 세브란스병원에서 코로나19 중증 환자에 혈장 치료를 시도해 2명이 완쾌했다.
정 본부장은 "수혈학회와 감염학회 전문가 의견과 혈액 관련 안전소위원회 심의를 받아 지침을 확정했다"며 "지침은 회복기 환자의 혈장을 채혈할 때 어떤 것을 확인하고, 검사해야 하는지 등 안전성에 관한 내용"이라고 설명했다.
이어 그는 "다만 혈장치료를 어느 환자에 어떻게 적용할지에 대해서는 전문가들 사이에 이견이 있다. 안전한 혈장을 확보한 후 그것을 어떻게 투약할지에 대해서는 담당 주치의와 의료기관들의 진료에 대한 방침이 필요한 상황이어서 의견을 모으고 있다"고 덧붙였다.
현재 메르스, 코로나19 등 치료제가 없는 신종 감염병 치료에 혈장 치료가 시도되지만, 의료계 일각에선 과학적으로 뚜렷한 근거가 없는 데다 큰 효과를 내지도 못할 수 있다는 점에서 우려를 제기하고 있다.